Scuba Diving Physic
PHYSIC
PRESSURE IN SALT WATER
- AT 20M THE PRESSURE IS 3 BAR
FORMULA:
20M / 10
+1 = 3BAR
SW ATM
PRESSURE
PRESSURE IN FRESH
WATER
- AT 20M THE PRESSURE IS 2.94BAR
FORMULA
20M / 10.3 +1 =2.94 BAR
FW ATM
PRESSURE
GAUGE
PRESSURE VS TOTAL/AMBIENT PRESSURE
- THE GAUGE PRESSURE IS THE AMBIENT PRESSURE
WITHOUT THE SURFACE PRESSURE
FORMULA GAUGE PRESSURE
20M / 10 =
2BAR
SW
20M / 10.3 = 1.94BAR
FW
- THE TOTAL OR AMBIENT PRESSURE IS THE SAME,
WE COUNT THE PRESSURE FROM THE SURFACE
FORMULA
20M / 10 +1 =
3BAR
SW SURFACE
PRESSURE
20M / 10.3 +1 =
2.94 BAR
FW SURFACE
PRESSURE
TEMPERATURE CHANGES ( CHARLES’ LAW)
- IF TEMPERATURE INCREASE, THE PRESSURE
INCREASE
- IF TEMPERATURE DECREASE, THE PRESSURE
DECREASE
- THE PRESSURE INCREASE OR DECREASE FROM 0.6
BAR PER DEGRES
FORMULA
FILL TANK TO 200BAR
TEMPERATURE OF THE TANK IS 40C WHEN FULL
THE NEXT MORNING THE
TANK TEMPERATURE IS 30C
CALCULATON:
40C – 30C = 10C
10C X 0.6 = 6 BAR
200BAR – 6 BAR = 194
BAR
AIR COMSUPTION
- THE DEEPER YOU GO THE MORE AIR YOU USE
- SHE SHALLOWER YOU YO THE LESS AIR YOU USE
FORMULA
PUT ON PAPER LIKE
THIS:
- CHANGE THE METERS TO BAR (BE CAREFULL IF
FRESH OR SALT WATER)
- BRING YOUR COMSUPTION TO THE SURFACE (
LESS SO DIVIDE)
- BRING YOUR COMSUPTION DOWN (MORE SO TIME)
- FINISH
- ALWAYS
BRING IT TO THE SURFACE 1ST
VOLUME CHANGES (BOYLE’S LAW)
- IF THE PRESSURE INCREASE, THE VOLUME
DECREASE
- IF THE PRESSURE DECREASE, THE VOLUME
INCREASE
FORMULA:
PUT ON PAPER LIKE
THIS:
- CHANGE THE METERS IN BAR (BE CAREFULL OF
FRESH OR SALT WATER)
- BRING YOUR VOLUME TO THE SURFACE ( MORE SO
TIME)
- BRING YOUR VOLUME DOWN ( LESS SO DIVIDE)
- FINISH
- ALWAYS
BRING TO THE SURFACE 1ST
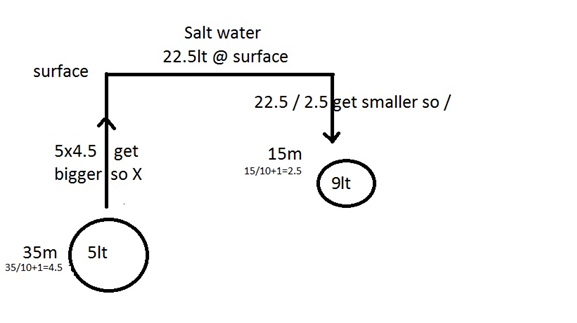
FRESH WATER:
ANSWER
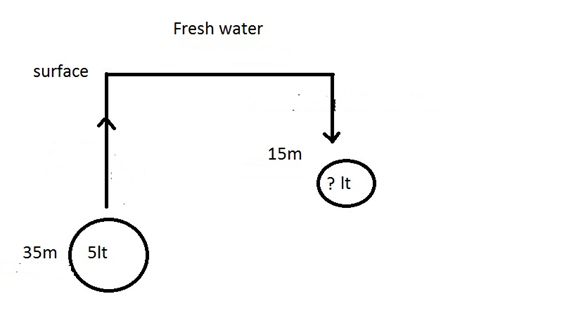
WEIGHT OF WATER
- 1LT OF FRESH WATER WEIGHTS 1.0 KG
- 1LT OF SALT WATER WEIGHTS 1.03 KG
FORMULA
10LT FW X 1 = 1KG
10LT SW X 1.03 =
1.03KG
ARCHIMEDE’S PRINCIPLE
- IF YOU PLACE AN OBJECT IN THE WATER, THIS
OBJECT WILL DISPLACE WATER.
- IF THE WEIGHT OF THE WATER DISPLACED IS
LESS THAN THE WEIGHT OF THE OBJECT, THE OBJECT WILL SINK (COIN IN WATER)
- IF THE WEIGHT OF THE WATER DISPLACED IS
MORE THE THE WEIGHT OF THE OBJECT, THE OBJECT WILL FLOAT
- IF THE WEIGHT OF THE WATER DISPLACES IS
THE SAME WEIGHT AS THE OBJECT, THE OBJECT WILL BE NEUTRAL
- BE CAREFULL IF THE OBJECT IS IN FRESH OR
SALT WATER
- YOU NEED TO KNOW: THE WEIGHT OF THE
OBJECT (KG), THE VOLUME OF WATER DISPLACED (LT) AND IF IN FRESH OR SALT
WATER
- IF YOU DON’T HAVE THESE NUMBERS, YOU
CANNOT CALCULATE!!!
FORMULA:
- ALWAYS PUT ON PAPER AND WRITE IF KG OR
LITERS
- YOU CANNOT DIVIDE KG BY LT
- CHANGE THE WATER(LT) IN KG
- MINUS YOUR RESULT TO THE WEIGHT OF THE
OBJECT
- IF YOU FIND – IN FRONT OF THE NUMBER, THE
OBJECT SINK(NEGATIVE BUOYANCY)
- TO FIND HOW MUCH AIR YOU NEED TO ADD IN
LIFTING BAG, CHANGE THE KG IN LITER AGAIN AND YOU GET YOUR ANSWER
- BE AWARE OF FRESH OR SALT WATER
ANSWER:
PARTIAL PRESSURE ( DALTON’S LAW)
- IN A MIXTURE OF GASES, THE TOTAL PRESSURE
OF THE MIXTURE IS EQUAL TO THE SUM OF THE 2 GASES
FORMULA:
IN AIR WE HAVE O2 AND
N2 OR SOME OTHER GASES WHO ARE TO SMALL TO BE COUNTED
O2 AROUND 21%
N2 AROUND 79%
AT THE SURFACE , THE
PRESSURE IS 1 BAR
THE TOTAL OF O2 AND N2
HASE TO BE EQUAL TO 1
TO FIND THE PARTIAL
PRESSURE, DIVIDE THE % BY 100
SURFACE WITH AIR 1 BAR
TOTAL PRESSURE
EXEMPLE: 21%
02 / 100 = 0.21 PPO2
+79% N2/ 100 = +0.79
PPN2
------- -------------
TOTAL 100% 1 BAR
10M WITH AIR SW 2 BAR
TOTAL PRESSURE
EXEMPLE: 21%
02 / 100 = 0.21 PPO2 X2 = 0.42 PP02
+79% N2/ 100 = +0.79
PPN2 X2 = +1.58 PP02
------- -------------
------------
TOTAL 100% 1 BAR 2BAR
- WHY WE NEED TO KNOW THE PARTIAL PRESSURE
- O2 IS TOXIC AT 1.4 PPO2 à CONVULTIONS
- N2 IS TOXIC AT 2.23PPN2 (18.3M) à NITROGEN NARCOSIS
HOW TO CALCULATE THE
MAX DEPTH WITH PPO2:
MAX DEPTH = 14 EMERGENCY
DEPTH 16
---- -10 CONTENGENCY
DEPTH --- - 10
FO2 FO2
EXEMPLE: DIVING WITH AIR 21%O2
14 16
--- -10 = 56.6M --- -10 = 66.19M 0.21 0.21
EXEMPLE: DIVING WITH
NITROX 32%
14 16
--- -10 = 33.75M --- -10 = 40M 0.32 0.32
HENRY’S LAW
- HENRY’S LAW STATE THAT WE ABSORB NITROGEN
TO TRY TO EQUILIZE THE AMBIENT PRESSURE WITH OUR INSIDE BODY PRESSURE
- THE DEEPER WE GO THE MORE DENSE THE AIR IS
SO WE ABSORB NITROGEN FASTER
- WE ABSORB NITROGEN UNTIL OUR BODY IS
SATURATED WITH N2 (NON DECOMPRESSION LIMIT)
- WHEN WE COME UP TO THE SURFACE, OUR N2
PRESSURE INSIDE OUR BODY IS HIGHER THEN THE AMBIENT PRESSURE SO WE RELEASE
N2
- WE ABSORB N2 MUCH FASTER THEN WE RELEASE
IT.
LIGHT
- LIGHT IS REFLECTED ON THE SURFACE OF THE
WATER
- THAN IT IS DIFFUSE AND ABSORB IN THE WATER
- BECAUSE OF THAT, WE LOOSE COLORS
- 1ST IS RED, THAN ORANGE, THAN
YELLOW, THAN GREEN, THAN BLUE
- OUR BLOOD AT 3M IS GREEN AND DARK BROWN AT
10M
- WE NEED LIGHT OF FLASHES TO SEE THE REAL
COLOR UNDERWATER
LIGHT à REFRACTION
- UNDERWATER, OBJECTS LOOKS BIGGER BY 33%
AND CLOSER BY 25%
- THIS IS CAUSE BY WATER AND LIGHT WHO
TRAVEL SLOWER IN THE WATER.
LIGHT à TURBIDITY
- TURBIDITY CAUSES OBJECT TO LOOK FARTHER
AWAY THAN THEY REALLY ARE.
SOUND
- SOUND TRAVEL ABOUT 4X FASTER IN THE WATER
- IT IS DIFFICULT TO KNOW WHERE THE SOUND
COME FROM
- OUR BRAIN THINK THAT THE SOUND COME FROM
OVERHEAD BECAUSE THE SOUND ARRIVES IN BOTH EARS AT THE SAME TIME
HEAT
- WE LOOSE HEAT 25X FASTER IN WATER
- THE BIGGEST LOOSE IS BY CONDUCTION( THE
WATER TOUCH THE SKIN AND DICIPATE THE HEAT)
- USE A WETSUIT TO PROTECT AGAINST IT.
- 75% OF THIS HEAT GOES AWAY BY THE HEAD, SO
IF YOU FEEL COLD PUT A HOOD.
.jpeg)









No comments:
Post a Comment